Project A01
Mechanism of prokaryotic specificity of ADEP antibiotics
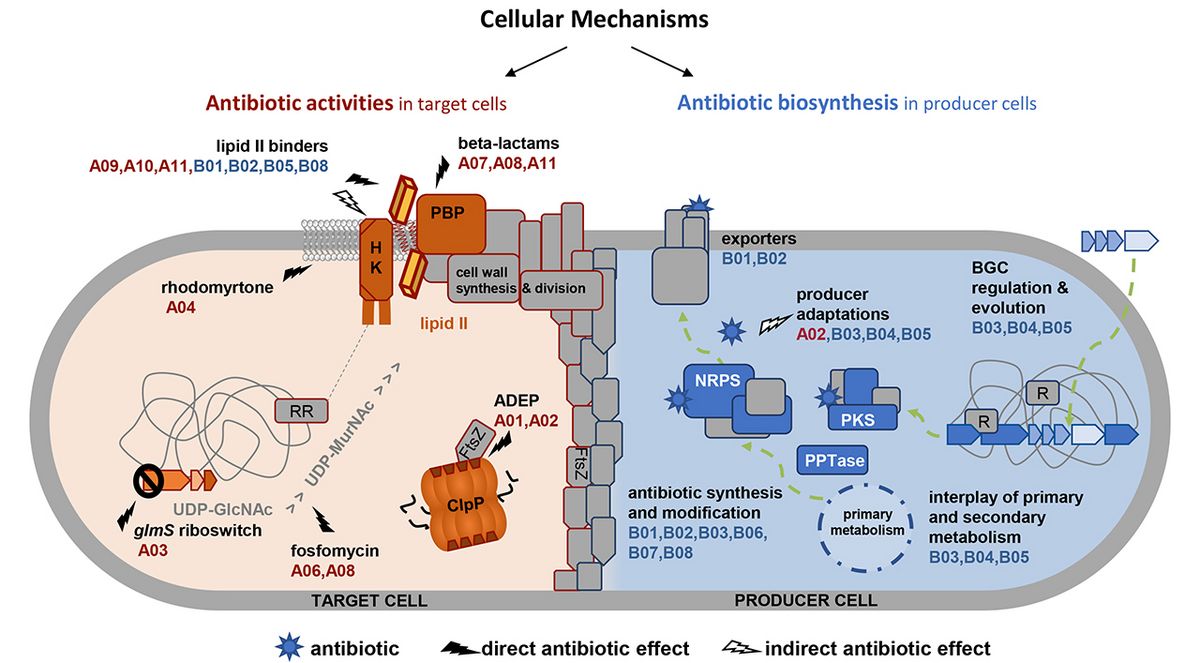
Antibacterial acyldepsipeptides of the ADEP class have a unique bactericidal mechanism of action by targeting ClpP, the proteolytic core of the caseinolytic protease. ClpP is broadly conserved in bacteria, mitochondria, and chloroplasts. Although ADEP can kill intracellular bacteria and, thus, enters eukaryotic cells, and although it was shown to deregulate mitochondrial ClpP in vitro, ADEP is less toxic to eukaryotic than prokaryotic cells. In this project, the reason for this prokaryotic specificity is investigated.

Principal Investigator
Interfakultäres Institut für Mikrobiologie und Infektionsmedizin
Mikrobielle Wirkstoffe
Eberhard Karls Universität Tübingen
Auf der Morgenstelle 28, 72076 Tübingen
+49 (0)7071 29-74706
heike.broetz-oesterheltuni-tuebingen.de
Project A02
Disturbing bacterial morphogenesis by antibiotic action
Antibiotic acyldepsipeptides (ADEPs) exert potent antibacterial activity against many Gram-positive pathogens by deregulating the bacterial Clp protease. The mode of killing is species-dependent, either by inhibiting native Clp functions or by activating the protease for deregulated protein degradation. With regard to protease activation, the bacterial cell division protein FtsZ is a preferred degradation target in Bacillus subtilis and Staphylococcus aureus and both species depend on FtsZ for viability. In the producer genus Streptomyces, FtsZ is not essential, but it is important for propagation via sporulation, and we here investigate the effect of ADEP on the multicellular life cycle of these bacteria.

Principal Investigator
Interfakultäres Institut für Mikrobiologie und Infektionsmedizin
Mikrobielle Wirkstoffe
Eberhard Karls Universität Tübingen
Auf der Morgenstelle 28, 72076 Tübingen
+49 (0)7071 29-74721
peter.sassuni-tuebingen.de
Project A03
Impact of carba-glucosamine on bacterial cell metabolism
The treatment of Bacillus subtilis and Staphylococcus aureus with carba-D-glucosamine (CGlcN) induces bacterial growth inhibition. This inhibition has been found to depend on the presence of a specific phosphoenolypyruvate-phosphosugar transferase system. This suggests that upon bacterial uptake, CGlcN is transformed into carba-D-glucosamine-6-phosphate (CGlcN6P), which has been shown to activate the glmS riboswitch. This activation leads to a reduced expression of the GlmS protein, a key enzyme for the synthesis of cell wall precursors, and consequently to bacterial growth inhibition. Our goal is to analyze the detailed molecular impact of CGlcN on cellular metabolism and growth.

Principal Investigator
LIMES Institut
Rheinischen Friedrich-Wilhelms-Universität Bonn
Gerhard-Domagk-Str. 1, 53121 Bonn
+49 (0)228 73-4808
gmayeruni-bonn.de
Project A04
Mode of action of rhodomyrtone (Rom) and synthesized derivatives and studying the resistance mechanism mediated by FarE / FarR
Rhodomyrtone (Rom) is an antibiotic that is active against many multi-resistant Gram-positive bacteria. A Rom-resistant Staphylococcus aureus mutant (RomR) has been isolated. Our preliminary studies suggest that FarR (a regulator) and FarE (a proposed fatty acid efflux pump) are causative for the resistance. Here, we want to study how FarE and FarR mediate resistance to Rom and probably other antibiotics. The chemical synthesis of Rom-derivatives is crucial to unravel the FarE substrate specificity.

Principal Investigators
Interfakultäres Institut für Mikrobiologie und Infektionsmedizin
Mikrobielle Genetik
Eberhard Karls Universität Tübingen
Auf der Morgenstelle 28, 72076 Tübingen
+49 (0)7071 29-74128
friedrich.goetzuni-tuebingen.de
Institut für Organische Chemie
Eberhard Karls Universität Tübingen
Auf der Morgenstelle 18, 72076 Tübingen
+49 (0)7071 29-75247
martin.e.maieruni-tuebingen.de
Project A06
Cellular responses to the antibiotic fosfomycin
We are investigating the cellular responses of bacteria to the antibiotic fosfomycin. Our goal is to better understand the synergistic actions often observed with fosfomycin in combination with other drugs. Applying growth inhibition studies, cell viability testing, as well as metabolomics, transcriptomics and proteomics, we aim at elucidating the responses of Staphylococcus aureus and Pseudomonas aeruginosa, two clinically relevant human pathogens, and the model organisms Escherichia coli and Bacillus subtilis to fosfomycin alone and in combination with synergistically acting antibiotics of various classes.

Principal Investigator
Interfakultäres Institut für Mikrobiologie und Infektionsmedizin
Mikrobiologie / Biotechnologie
Eberhard Karls Universität Tübingen
Auf der Morgenstelle 28, 72076 Tübingen
+49 (0)7071 29-74645
christoph.mayeruni-tuebingen.de
Project A07
Mechanisms of β-lactam induced persistence in chlamydiae
The obligate intracellular Chlamydiaceae lack a cell wall but synthesize a transient peptidoglycan ring for cell division. Moreover, the otherwise bactericidal β-lactams do not kill Chlamydiaceae but lead to cell division arrest and reversible persistence. Our goal is to elucidate the mechanisms that underlie β-lactam triggered arrest of cell division accompanied by formation of non-dividing persisting cells inside the host. We will use the reduced-genome Chlamydiaceae as model systems to study cellular activities of β-lactams beyond mere inhibition of penicillin-binding proteins. We will also learn how β-lactams can act bacteriostatically rather than bactericidal.

Principal Investigator
Institut für Pharmazeutische Mikrobiologie
Rheinische Friedrich-Wilhelms-Universität Bonn, Universitätsklinikum Bonn
Meckenheimer Allee 168, 53115 Bonn
+49 (0)228 73-4637
bhenrichuni-bonn.de
Project A08
Mode of action of cell wall biosynthesis inhibitors in Wolbachia spp
We will elucidate the connection between the lipid II cycle and cell division in the obligate intracellular Wolbachia, a genus of Gram-negative alpha-proteobacteria thought to have no cell wall. Using fosfomycin, the impact of inhibiting MurA on cell division will be studied at the cellular and molecular levels. We will employ transcriptomics and proteomics to clarify how fosfomycin depletes these endobacteria from host cells and how they respond to blocking of the lipid II cycle, including its recycling. Using labeled precursors, Wolbachia will be probed for a rudimentary peptidoglycan and the role of lipid II in interacting with or coordinating the cell division machinery (e.g. MreB or FtsZ) will be investigated.

Principal Investigator
Institut für Medizinische Mikrobiologie, Immunologie und Parasitologie
Universitätsklinikum Bonn
Venusberg-Campus 1, 53127 Bonn
+49 (0)228 287-11207
kenneth.pfarrukbonn.de
Project A09
Recognition mechanisms of guanidine-containing lipopeptide antibiotics for cell wall precursor targets
The guanidine-containing lipopeptide antibiotics empedopeptin, plusbacins, and tripropeptins exhibit considerable structural similarity, but show significant differences in potency. This project aims to arrive at a better understanding of the molecular and cellular reasons underlying the observed differences in potency by determining their binding modes to lipid II and putative additional interactions with the cytoplasmic membrane.

Principal Investigators
Pharmazeutisches Institut
Abteilung Pharmazeutische Biologie
Eberhard Karls Universität Tübingen
Auf der Morgenstelle 8, 72076 Tübingen
+49 (0)7071 29-76970
harald.grossuni-tuebingen.de
Institut für Pharmazeutische Mikrobiologie
Rheinische Friedrich-Wilhelms-Universität Bonn,
Universitätsklinikum Bonn
Meckenheimer Allee 168, 53115 Bonn
+49 (0)228 73-5688
tschneideruni-bonn.de
Project A10
From primary target inhibition to collapse of the cell envelope biosynthetic network
Binding bactoprenyl (C55-P)-coupled cell envelope precursors, such as lipid II, has proven an attractive antibiotic strategy. Compared to other lipid II binding antibiotics, the recently discovered teixobactin appears unique. Teixobactin binds to multiple C55P-containing cell wall lipid intermediates, shows increased and more rapid bacteriolytic activity, and appears less prone to resistance development. In this project, we will investigate the multifactorial secondary effects triggered by teixobactin and other lipid II binding antibiotics that go beyond mere target blocking, finally resulting in collapse of biosynthetic networks, and how these effects differentially relate to the propensity for developing resistance.

Principal Investigator
Institut für Pharmazeutische Mikrobiologie
Rheinische Friedrich-Wilhelms-Universität Bonn,
Universitätsklinikum Bonn
Meckenheimer Allee 168, 53115 Bonn
+49 (0)228 73-5688
tschneideruni-bonn.de
Project A11
Impact of antibiotics on the spatio-temporal organisation of the cell wall biosynthesis machinery
We study the downstream effects of different antibiotics on the spatio-temporal organization of the membrane-bound cell wall biosynthesis machinery and how this contributes to drug potency. Antibiotic and target localizations will simultaneously be monitored in Staphylococcus aureus by advanced fluorescence microscopy while monitoring membrane integrity and cell viability. The effects of antibiotics with varying degree of membrane activity will be compared. We will quantify interactions between components of the cell wall biosynthesis machinery and antibiotics in model membranes as a function of membrane properties.
Principal Investigators
Institut für Pharmazeutische Mikrobiologie
Rheinische Friedrich-Wilhelms-Universität Bonn
Universitätsklinikum Bonn
Meckenheimer Allee 168, 53115 Bonn
+49 (0)228 73-5247
greinuni-bonn.de
Institut für Physikalische und Theoretische Chemie
Abteilung Biophysikalische Chemie
Rheinische Friedrich-Wilhelms-Universität Bonn
Wegeler Str. 12, 53115 Bonn
+49 (0)228 73-2262
u.kubitscheckuni-bonn.de
- Project A01
- Project A02
- Project A03
- Project A04
- Project A06
- Project A07
- Project A08
- Project A09
- Project A10
- Project A11