New TRR-paper! Structural insights into ClpP protease side exit pore-opening by a pH drop coupled with substrate hydrolysis.
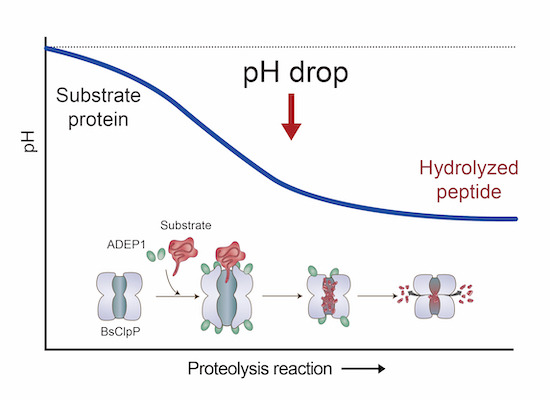
The catalytic cycle of ClpP is dynamic and ADEP helped to shed further light on the details and intermittent stages: Delineating the mechanisms of substrate engagement and product release is essential for understanding the role of the conserved ClpP protease in protein homeostasis and developing antibiotics. This study shows that the opening of ClpP side exit pores is coupled to a pH decrease caused by peptide accumulation in the proteolytic chamber. Heike Brötz-Oesterhelt is grateful for the great collaboration with Hyun Kyu Song and his team at Korea University.
The research paper can be accessed under:
Congratulations to all authors!

